Abstract
INTRODUCTION:
Haploidentical stem cell transplants are increasingly performed for a wide variety of malignant and non-malignant hematological conditions. Epstein - Barr virus (EBV) viremia and subsequent development of Post-Transplant Lymphoproliferative Disorder (PTLD) are potential adverse sequelae of the transplantation process and associated immunosuppression. Traditionally, haploidentical transplants have been associated with increased risk of EBV reactivation and subsequent PTLD. This was largely due to graft versus host disease (GVHD) prophylaxis protocols using intense immunosuppression with either Ex-vivo T-cell depletion or in-vivo T- cell depletion with anti-thymocyte globulin (ATG) and Alemtuzumab. However, most haploidentical transplants in recent years have utilized post-transplant cyclophosphamide (PTCy) for GVHD prophylaxis. Rituximab has been used for preemptive treatment of EBV viremia/PTLD, but there is scarce data on its use in this setting, as well as the risk factors and outcomes of EBV viremia associated with haploidentical transplants with PTCy.
RESULTS:
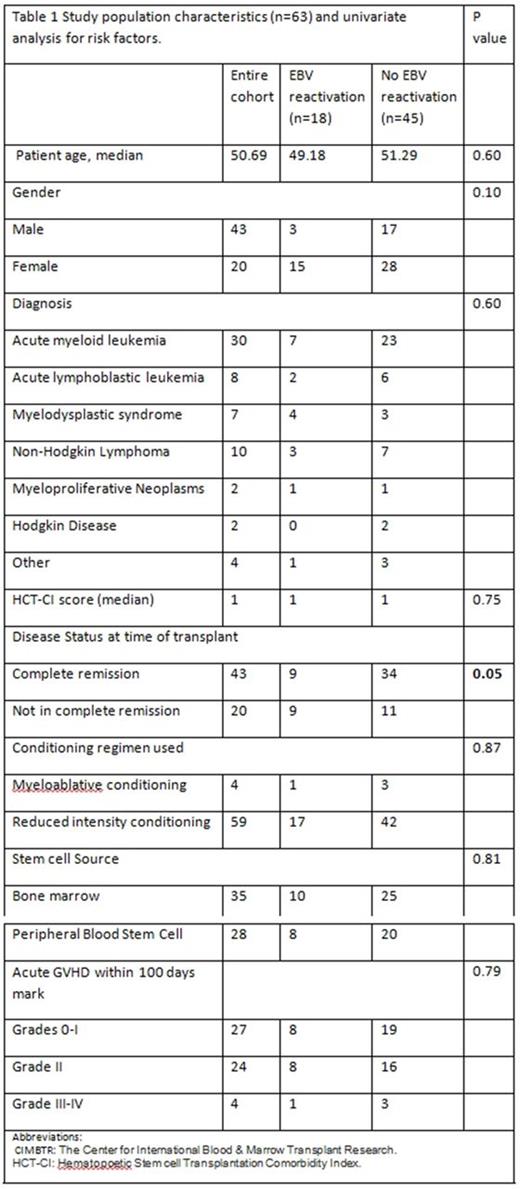
A total of 63 patients with hematological malignancies received haploidentical transplant at our institution from 2013 from 2016. Patient characteristics are summarized in Table 1.
All patients received EBV monitoring using plasma viral copies once weekly starting 20 days post-transplant. EBV monitoring continued for 6 months after immunosuppression ended or when CD4 count >200. Among these 63 patients, 18 (28.5%) became EBV DNA positive upon follow-up. Per institutional protocol, EBV titer was confirmed by repeat testing when viral load was 1000 copies/mL and treatment with Rituximab was initiated if repeat titer was >1000 copies/ml.
The median time to detection of EBV viremia from the time of transplant was 99 days (range: 20-277). The median peak level for the EBV reactivation cohort was 7650 viral copies /ml. The median time from detection of viremia to peak viremia was 1.5 days (range 0-253).
8 of the 18 (44.4%) patients that developed EBV viremia required Rituximab due to rising or persistent EBV viremia. The median EBV level at initiation of therapy was 31,000 copies/ml for the 8 patients that received Rituximab treatment. EBV seronegativity was achieved in 6 patients (75%) with treatment at a median of 13.5 days (range 3-26). The median number of Rituximab cycles administered was 1.5 (range: 1-16). None of the patients in either cohort developed PTLD or other EBV associated diseases.
A univariate analysis was performed comparing EBV reactivation cohort and no EBV reactivation cohort to look for risk factors predisposing to EBV reactivation (Table 1) There were no statistically significant differences seen in the variables studied other than patients not in complete remission at the time of transplant were statistically more likely to develop EBV viremia. 9 out of 18 patients (50%) that developed EBV viremia were not in complete remission at the time of transplant, whereas 11 out of the 45 patients (24.44 %) that did not develop EBV viremia were in an incomplete remission at the time of transplant (p value: 0.05) (Table 1).
With a median follow up of469 days, there were a total of 22 deaths among the 45 (48.9 %) patients that had no viremia, and 8 deaths amongst the 18 (44.4 %) patients that had viremia (p value: 0.75). There was no statistically significant difference in graft versus host disease (GVHD) incidence (p value: 0.79) or overall survival (555 days for patients with EBV reactivation versus not reached for patients without EBV reactivation, p value: 0.45) between these two groups (p value: 0.79)
CONCLUSIONS:
In this retrospective study assessing risk factors and outcomes of EBV viremia in patients following haploidentical transplants, we observed a 28.5 percent incidence of EBV viremia. Rituximab was effective in preemptive treatment of EBV viremia. However, no cases of PTLD were observed, even in those patients with sustained elevation in EBV titers. It is unclear how to manage asymptomatic elevations in viral titers after allogeneic transplantation in patients who are otherwise at low risk for development of PTLD. Previous data suggests that even high levels of EBV viremia lacks specificity for prediction of PTLD in post-transplant setting. Given these results, the utility of routine monitoring of EBV viral load after haploidentical transplant should be questioned in those who have received PTCy.
Lin: Jazz Pharmaceuticals: Consultancy. Ganguly: Amgen: Other: Advisory Board; Seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal